Non Invasive Prenatal Testing (NIPT)
NON INVASIVE PRENATAL TESTING (NIPT)
During pregnancy, parts of the fetal DNA enter the maternal blood and can be detected from the 7th week of pregnancy. The amount of circulating fetal DNA increases with increasing duration of pregnancy and is sufficient to ensure a highly specific and sensitive NIPT screening result after the 10th week of pregnancy. Through the blood taken from the mother, changes in the genetic structure of the unborn child, i.e. chromosomal disorders, can be detected.
This test is a safe examination method for the unborn child. PrenatalSafe® can help alleviate your worries and fears about certain health problems of the baby.
Our NIPT tests have clinical performance data in millions of pregnant women published in clinical journals. With comprehensive genetic information, a broad screening test includes the following numerical and/or structural abnormalities, microdeletions, de novo mutations, fetal RH incompatibility, genetic disorders at the level of individual genes in the fetus, and more by analysing cell-free foetal DNA (cffDNA) circulating in maternal blood.
Why choose PrenatalSafe for a NIPT test ?
What can be investigated with NIPT ?
What do NIPT tests check for?
Chromosomal aneuploidies: These are chromosomal abnormalities characterised by an altered number of chromosomes, i.e. more or less than the standard number of chromosomes. For example, we speak of trisomy when there is an extra chromosome, or monosomy when one chromosome is lost. Chromosomal trisomy Aneuploidies in the test fetus; for example Down's syndrome, Edwards' syndrome, Pateu's syndrome, monosomy X, trisomy X, Klinefelter's syndrome and Jacobs' syndrome; in addition, testing for all chromosomal monosomies and trisomies is carried out.
TRISOMY 21
It is caused by the presence of an extra copy of the chromosome and is also known as Down's syndrome. It is the most common genetic cause of mental retardation. It is estimated that trisomy 21 affects about 1 in 700 newborns.
TRISOMY 18
It is caused by the presence of an extra copy of chromosome 18. Edwards syndrome is associated with a high abortion rate. It causes severe mental retardation. Babies with trisomy 18 often have congenital heart defects and other pathological conditions that shorten their life expectancy. It is estimated that trisomy 18 occurs in 1/5,000 births.
TRISOMY 13
It is caused by the presence of an extra copy of the 13th chromosome. It is also known as Patau syndrome and is highly associated with miscarriages. Babies with trisomy 13 have multiple heart defects, severe cognitive impairment and developmental problems. They do not survive beyond the first few months of life. It is a much rarer condition than Down syndrome, which occurs in about 16,000 newborns.
Structural chromosomal abnormalities: The test examines both structural and numerical abnormalities in the foetal genome and provides information on both karyotypic assesment and insertions (duplications) and deletions (deletions) in chromosomes throughout the genome.
Microdeletion syndromes: Provides the ability to scan for clinically important microdeletions.
Foetal RH Incompatibility test: The RhSafe ® test is a non-invasive prenatal test that consists in genotyping the fetal RHD from the maternal plasma of RhD - women and detects the presence or deletion of the RHD gene ( fetal RhD - ) or RHD gene (fetal RhD) in the plasma. PrenatalSafe ® can be combined with the RhSafe ® test, a non-invasive prenatal test that allows the Fetal Rh(D) factor to be determined by analysing fetal DNA isolated from a pregnant woman's blood sample. The RhSafe ® test is optional and is performed on request in Rh(D)-negative pregnant women with an Rh(D)-positive partner. The RhSafe ® test can determine when immunoprophylaxis is unnecessary and can be avoided in pregnant women: Pregnant Rh- and foetus Rh-.
HOW DOES PRENATALSAFE WORK?

The amount of circulating foetal DNA from 9-10 weeks of gestation is sufficient to ensure high specificity and sensitivity of the test. The test is performed starting from a simple blood sample from the mother whose gestational age is at least 10 weeks. Laboratory analysis is used to isolate the free circulating foetal DNA from the plasma component of the maternal blood.
The next step is an advanced technological process: chromosomal regions of the circulating fetal DNA are sequenced at a high read depth (~30 million sequences) of the entire fetal genome by the innovative Dense Parallel Sequencing (MPS) technology, based on Illumina's market-leading Next Generation Sequencing (NGS) technology. The chromosomal sequences are finally measured by bioinformatic analysis to determine the presence of any fetal chromosomal aneuploidies. The failure rate of the test is very low (0.3%).
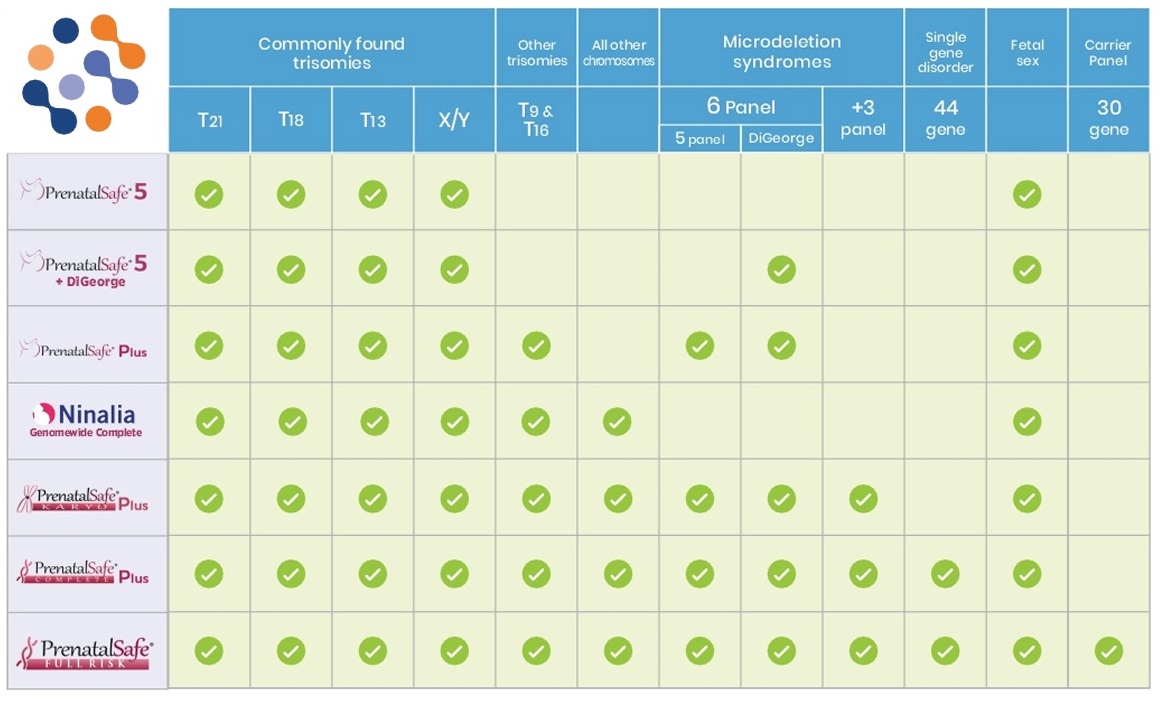
- PrenatalSafe ® is offered by the Eurofins Genoma Group laboratories. You have the option to choose between six levels of prenatal screening, each with a different level of detail. It is the most technologically advanced and comprehensive non-invasive prenatal screening test available today. Today, it is possible to screen for additional chromosomal abnormalities (autosomal abnormalities, all deletions and duplications above 7 mb) with PrenatalSafe® Karyo. The available non-invasive prenatal tests look for aneuploidies and microdeletions. PrenatalSafe® Karyo also provides karyotype-level information by screening for rare aneuploidies and segmental chromosomal imbalances (gains and losses) on each chromosome in the fetal genome. Click here to explore PrenatalSafe test options.
- GeneSafe™ goes even further. Through cfDNA analysis from maternal plasma, several clinically significant and life-changing genetic disorders are detected that cannot be screened for with current NIPT technology. Click here to explore GeneSafe test options.
*Click here to reach us on WhatsApp
**You can check the links below for more details;