OncoNext

OncoNext is a new department of Genoma Group dedicated entirely to oncology. This department consists of a multidisciplinary professional team specialized in molecular biology and genetics applied to the study of solid tumors. OncoNext tests are designed to detect somatic and germinal mutations from blood or tissue biopsy samples.
The promotion of the study of germinal mutations related to hereditary solid tumors over the years has not only improved the prognosis of potential pathologies through personalized monitoring programs but has also allowed for the enhancement of patient treatment processes by managing targeted pharmacological therapies when necessary.
The solid use of molecular biology to determine the presence of somatic mutations in oncology, as well as centralizing the relationship between the patient and the healthcare provider, has made it possible to better meet individual needs and enhance personal treatment methods through the development and application of "smart drugs."

ONCONEXT RISK™ includes tests dedicated to examining germline mutations, which are present in all of an individual's cells and can be inherited through generations. These mutations are believed to play a role in the development of hereditary tumors.
The mutations analyzed are localized in genes that encode tumor suppressor proteins (which regulate cell proliferation, DNA repair, and apoptosis). These mutations limit the biological functions of the proteins, restricting tumor-suppressing activities.
Individuals carrying these mutations are known to have a higher susceptibility to developing tumor pathologies.
Due to mutagenic factors encountered daily, tumor-suppressing activities may be at greater risk than in the general population. Additionally, exposure to mutagenic agents may allow potential tumor-suppressing genes derived from proto-oncogenes (which function in cell cycle progression and cell proliferation) to freely activate.
OncoNext® Risk – Oncoscreening CompleteBreastColon + Gastric + Melanoma + Ovarian + Pancreas + Prostate + Thyroid) NGS Screening (82 genes)
(Test Result: 30 days, Sample Type: EDTA Tube)
Genes: AIP, APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDC73, CDH1, CDK4, CDKN1B, CDKN2A, CEBPA, CHEK2, DICER1, EGFR, EPCAM, EXT1, EXT2, FH, FLCN, GALNT12, GATA2, GPC3, GREM1, HOXB13, HRAS, KIT, MAX, MEN1, MET, MITF, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, NF2, NSD1, PALB2, PHOX2B, PMS1, PMS2, POLD1, PRF1, PRKAR1A, PTCH1, PTEN, RAD50, RAD51C, RAD51D, RB1, RET, RHBDF2, RUNX1, SBDS, SDHA, SDHAF2, SDHB, SDHC, SDHD, SMAD4, SMARCA4, SMARCB1, SMARCE1, STK11, SUFU, TMEM127, TP53, TSC1, TSC2, VHL, WT, CTNNA1, PDGFRA, PIK3CA, KRAS, TGFR2
OncoNext® Risk – Breast NGS Screening (13 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, PALB2, RAD51C, RAD51D, STK11, TP53, PTEN
OncoNext® Risk – Colon NGS Screening (19 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: APC, BMPR1A, CDH1, EPCAM, GREM1, MLH1, MSH2, MSH6, MUTYH, PMS2, PIK3CA, POLD1, PTEN, SMAD4, STK11, TP53, KRAS, PMS1, TGFBR2
OncoNext® Risk – Gastric NGS Screening (11 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: APC, BMPR1A, EPCAM, MLH1, MSH2, MSH6, PMS2, STK11, SMAD4, TP53, CTNNA1
OncoNext® Risk – Melanoma NGS Screening (8 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: BAP1, BRCA2, CDK4, CDKN2A, MITF, PTEN, RB1, TP53
OncoNext® Risk – Ovarian NGS Screening (15 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: BRCA1, BRCA2, BRIP1, EPCAM, MLH1, MSH2, MSH6, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, CHEK2, ATM
OncoNext® Risk – Pancreas NGS Screening (14 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: ATM, BMPR1A, BRCA1, BRCA2, CHECK2, CDKN2A, EPCAM, MLH1, MSH2, MSH6, PALB2, PMS2, STK11, TP53
OncoNext® Risk – Prostate NGS Screening (13 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PALB2, TP53, PMS2
OncoNext® Risk – Thyroid NGS Screening (1 gene)
(Test Result: 20 days, Sample Type: EDTA Tube)
Gene: RET
OncoNext® Risk BRCA (BRCA1, BRCA2) NGS Screening (2 genes)
(Test Result: 20 days, Sample Type: EDTA Tube)
Genes: BRCA1, BRCA2

ONCONEXT LIQUID™ CNV Test is a test based on next-generation sequencing (NGS) that analyzes circulating tumor DNA (ctDNA) in plasma samples collected from patients to detect large chromosomal gains and losses (Copy Number Variations – CNVs) in cancer cells' genomes. The identification of CNVs across the genome is a result of genomic instability (Copy Number Instability), a characteristic feature of cancer cells, and indicates the presence or progression of a tumor.
The ONCONEXT LIQUID™ CNV test is suitable for monitoring patients undergoing chemotherapy or immunotherapy, as well as for monitoring recurrence after tumor resection.
Developed by Genoma Group, the ONCONEXT LIQUID™ genetic tests are designed for the examination of somatic mutations in oncological patients or individuals at high risk by analyzing circulating tumor DNA (ctDNA) in peripheral blood.
ctDNA testing, also known as liquid biopsy, is one of the most innovative tools currently available for oncologists. With Onconext Liquid™, the liquid biopsy offered by Genoma Group, clinicians can accurately improve the diagnosis and prognosis of oncological patients and detect the presence of somatic mutations that could indicate tumor foci in high-risk individuals. This technology allows for very early detection.
Onconext Liquid™ does not replace the tissue biopsy, which remains the gold standard for the initial morphological/histological diagnosis and genomic profiling of pathology, but it validates and deepens the information obtained.
Onconext Liquid™ is also the best alternative test for cases where traditional biopsy is limited due to its invasive nature.
Onconext® Liquid Breast
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: AKT1, EGFR, ERBB2, ERBB3, ESR1, SF3B1, KRAS, PIK3CA, FBXW7, TP53
Onconext® Liquid Colon
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: ALK, ROS1, RET, BRAF, KRAS, NRAS, NTRK1, EGFR, ERBB2, NTRK3, APC, TP53
Onconext® Liquid Gastric
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: ERBB2, NTRK1, NTRK3
Onconext® Liquid Lung
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: ALK, BRAF, EGFR, KRAS, MET, NRAS, RET, ROS1, ERBB2, NTRK1, NTRK3
Onconext® Liquid Thyroid
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: ALK, KRAS, NRAS, HRAS, BRAF, PIK3CA, CTNNB1, RET, NTRK1, NTRK3
Onconext® Liquid 50 Genes
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
Genes: AKT1, EGFR, FLT3, KRAS, PDGFRA, ALK, ERBB2, GNA11, MAP2K1, PIK3CA, AR, ERBB3, GNAQ, MAP2K2, RAF1, ARAF, ESR1, GNAS, MET, RET, BRAF, FGFR1, HRAS, MTOR, ROS1, CHEK2, FGFR2, IDH1, NRAS, SF3B1, CTNNB1, FGFR3, IDH2, NTRK1, SMAD4, DDR2, FGFR4, KIT, NTRK3, SMO, APC, FBXW7, PTEN, TP53, CCND1, CCND2, CCND3, CDK4, CDK6, MYC, ERG, ETV1, RET
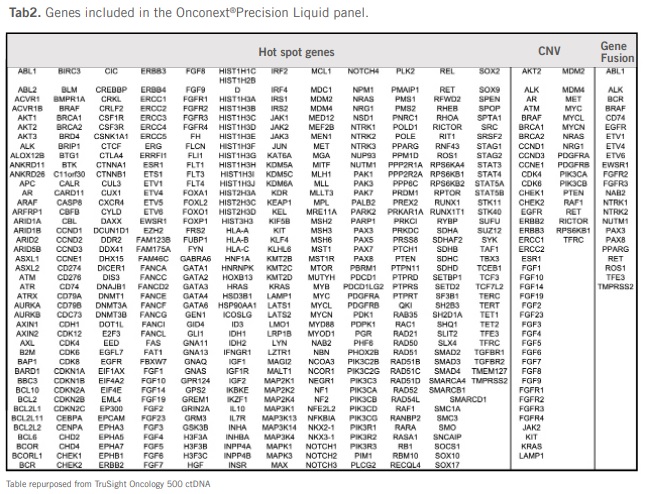
Onconext® Precision Liquid
(Test Method: NGS, Test Result: 20 days, Sample Type: Streck Tube)
DNA-based genomic profiling of >523 genes with recurrent hotspot mutations, 59 with focal acquisition or loss of CNVs, 23 fusion genes, 49 fusion driver genes, TMB.
Includes hotspots, CNVs, and fusions.
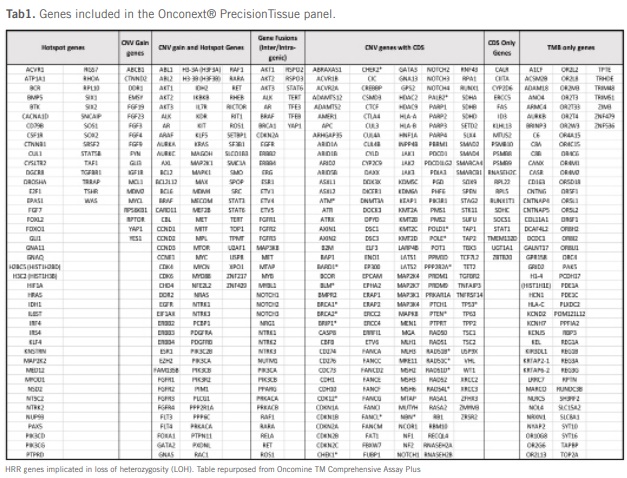
See Onconext® Precision Liquid Tab2 gene list.
Easilycare BLADDER: A test that evaluates the DNA methylation pattern of 15 biomarkers associated with urothelial carcinoma using a urine sample.
Signatera: The test measures circulating tumor DNA to detect and quantify residual tumor in the body (minimal residual disease).

ONCONEXT Tissue™ Genetic Tests are designed for oncological use to detect somatic mutations in tissue biopsy samples related to solid tumors. These tests use innovative Next-Generation Sequencing (NGS) technology, enabling highly accurate detection of somatic mutations even from small amounts of tumor tissue.
The simultaneous detection of different mutations allows for a better understanding of the tumor's genomic profile and facilitates the application of the most appropriate treatment.
ONCONEXT Tissue™ genetic tests are intended for oncological use, providing valuable information for various clinical and diagnostic purposes in oncology patients:
- Tumor profiling for accurate application: OncoNext Tissue™ Monitor can provide useful information for oncologists to create personalized treatment plans.
- Prognostic information provision
- Support for clinical trial eligibility: An additional function of OncoNext Tissue™ tests is to identify specific clinical trials the patient may qualify for based on the test results.
Onconext® Tissue Colon
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ALK, BRAF, ERBB2, KRAS, NRAS, RET, ROS1, EGFR, NTRK1, NTRK2, NTRK3
Onconext® Tissue Gastric
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ERBB2, NTRK1, NTRK3, NTRK2
Onconext® Tissue Lung
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ALK, BRAF, EGFR, ERBB2, KRAS, MET, NRAS, RET, ROS1, NTRK1, NTRK2, NTRK3
Onconext® Tissue Pancreas
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ALK, FGFR2, KRAS, RET, ROS1, BRAF, NTRK1/2/3
Onconext® Tissue Ovary
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: POL-E, TP53, PIK3CA, PTEN
Onconext® Tissue Thyroid
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ALK, BRAF, CTNNB1, HRAS, KRAS, NRAS, PIK3CA, RET, NTRK1, NTRK2, NTRK3
Onconext® Tissue BRCA
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: BRCA1 and BRCA2
Onconext® Tissue BRCA Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: BRCA1, BRCA2, and HRR
HRR Gene Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCD2, FANCL, MRE11, NBN, PALB2, POLD1, POLE, PPP2R2A, PTEN, RAD50, RAD51, RAD51B, RAD51C, RAD51D, RAD52, RAD54L, TP53, XRCC2 + KRAS, PIK3CA
Microsatellite Instability (MSI) (Bethesda Panel)
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Markers: BAT-25, BAT-26, CAT-25, MONO-27, NR-21, NR-22, NR-24, NR-27
Lung First Level Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: ALK, BRAF, EGFR, KRAS, MET, NTRK-1/2/3, RET, ROS1
BRAF Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Gene: BRAF V600-601
RAS Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: KRAS, NRAS, HRAS
RAS + BRAF Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: KRAS, NRAS, HRAS, BRAF (V600-601)
EGFR Panel
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Major mutations
Onconext® Tissue 50 Genes
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genes: AKT1, ALK, AR, BRAF, CDK4, CTNNB1, DDR2, EGFR, ERBB2, ERBB3, ERBB4, ESR1, FGFR2, FGFR3, GNA11, GNAQ, HRAS, IDH1, IDH2, JAK1, JAK2, JAK3, KIT, KRAS, MAP2K1, MAP2K2, MET, MTOR, NRAS, PDGFRA, PIK3CA, RAF1, RET, KT1, CCND1, CDK6, FGFR4, MET, MYC, MYCN, PDGFRA, AKT3, AXL, ERG, ETV1, ETV4, ETV5, NTRK1, NTRK2, NTRK3, PDGFRA, PPARG, ROS1
Onconext® Precision Tissue
(Test Method: NGS, Test Result: 20 days, Sample Type: Biopsy Material)
Genomic Profiling on DNA and RNA > 500 genes: 47 Fusion genes, TMB, 156 hotspots, 333 CNVs, BRCA1 and BRCA2, HRD, MSI, allowing for a measure of genomic instability, Genomic Instability Metric (GIM)
Includes hotspots, CNVs, and fusions.
See Onconext® Precision Tissue Tab1